Immunisation and vaccine-preventable diseases quarterly report
April to June 2022 (Q2)
An Official Statistics publication for Scotland
- Published
- 06 September 2022
- Type
- Statistical report
- Author
- Public Health Scotland
About this release
Our quarterly update
This release provides quarterly information on the following immunisations and vaccine-preventable diseases under surveillance in Scotland:
- diphtheria
- Haemophilus influenzae
- invasive pneumococcal disease
- measles
- meningococcal disease
- mumps
- pertussis
- poliomyelitis
- rotavirus
- rubella
- shingles
- tetanus
Next release
The next release of this publication will be 6 December 2022.
Main points
Vaccine-preventable disease
The number of reported cases of vaccine-preventable diseases in Scotland remains low and stable.
All vaccine-preventable diseases under surveillance have shown a notable reduction since early 2020.
This is likely to be a result of the social distancing measures and restrictions implemented in response to the COVID-19 pandemic.
Invasive bacterial diseases
Case numbers for invasive pneumococcal disease and meningococcal disease in the first half of 2022 were higher than those for the first half of 2021.
- There were 178 cases of pneumococcal disease compared with and 113 in 2021.
- There were 15 cases of meningococcal disease compared with five in 2021.
Cases reported in 2022, however, remain lower than the number of cases reported for the same period in each of the previous three years (2018 to 2020).
Case numbers for invasive Haemophilus influenzae in the first half of 2022 were:
- higher than those in the first half of 2020 and 2021, with 36 cases compared with 34 and 10 in 2020 and 2021, respectively
- slightly lower than for the same period in 2018 and 2019
Measles, mumps and rubella
No cases of measles have been reported between April and June this year.
There was one reported case of measles from the first quarter of 2022, which was imported from outwith the UK. This was the first case reported in Scotland since 2019.
There was one case of mumps reported in 2022 between April and June and a single laboratory-confirmed case was reported in 2021.
No cases of rubella have been reported in Scotland since 2017.
Pertussis
Two cases of pertussis have been reported in 2022, both between April and June.
Only four laboratory-confirmed cases were reported in 2021, a considerable decline from the 198 cases reported in 2020.
Results and commentary
Redirect
Diphtheria
Background information
Diphtheria is an acute bacterial infection affecting the upper respiratory tract or the skin.
It is caused by the diphtheria toxin produced by toxigenic strains of:
- Corynebacterium diphtheriae (C. diphtheria)
- Corynebacterium ulcerans (C. ulcerans)
- Corynebacterium pseudotuberculosis (C. pseudotuberculosis) – this is less common
The most common symptoms of diphtheria affecting the upper respiratory tract are membranous pharyngitis with fever, lymphadenopathy and upper respiratory tract soft tissue swelling ‘bull neck’ potentially leading to life-threatening airway obstruction.
Cutaneous diphtheria may cause pus-filled blisters on legs, hands and feet and ulceration of the skin.
In unvaccinated or partially vaccinated individuals, systemic absorption of the toxin can lead to late complications such as cardiac and neurological conditions and sometimes death.
Immunisation against diphtheria is offered to babies and children as part of the routine childhood immunisation schedule.
They receive:
- three doses of the 6-in-1 vaccine at 8, 12 and 16 weeks of age
- a 4-in-1 pre-school booster from 3 years 4 months old.
- a 3-in-1 teenage booster at around 14 years of age (S3)
Guidance on diphtheria immunisation is available in chapter 15 of the UKHSA’s green book.
Surveillance
Diphtheria is now rare in the UK because babies and children have been vaccinated against it since the 1940s.
Prior to the introduction of a vaccine there were up to 70,000 cases a year, causing around 5,000 deaths.
There have been no toxigenic strains of diphtheria reported in 2022.
Vaccine uptake statistics
Vaccine uptake statistics for children are published in our childhood immunisation statistics quarterly report.
Haemophilus influenzae
Background information
In 1992, following introduction of the Hib vaccine for young children, the number of H. influenzae type b cases fell dramatically, not only in the vaccinated group but also in older age groups.
Due to reduced carriage of the organism within the respiratory tract of vaccinated children, transmission to the wider community was effectively suppressed.
The addition of the Hib booster vaccine in 2006 to the childhood immunisation schedule, reduced case numbers further.
In Scotland, typing is conducted on cases with positive laboratory reports for H. influenzae in order that national trends in disease subtypes can be monitored.
Further enhanced surveillance is carried out for all H. influenzae cases identified in children under the age of 5 and type b strains across all age groups.
Surveillance update for April to June 2022
There were 21 invasive H. influenzae cases reported in the second quarter of 2022, bringing the total number of cases for the first half of the year to 36.
This is higher than the number of cases reported for the same period in 2021 (n=10) and 2020 (n=34), but slightly lower than the number of cases reported for the same period in 2019 (n=40) and 2018 (n=42).
This may indicate a return to pre-pandemic case levels.
Of the 36 cases reported in the first half of 2022:
- 19 were people aged over 40 years
- seven were people aged between 21 to 40 years
- three were in people aged between 5 and 20 years
- seven were children aged under 5 years old – three of whom were infants aged under one year
There were no deaths associated with H. influenzae reported in the first half of 2022.
Figure 2 demonstrates the epidemiological impact of the Hib vaccine, for those aged under five (routinely vaccinated group) and for all ages (including under-fives).
There was a marked decrease in cases from 1992 in all age groups, followed by a rise in case numbers in the early 2000s.
Case numbers decreased again following the introduction of the Hib booster vaccine, and figures have remained relatively stable since 2011.
Figure 3 presents laboratory reports by serotype, since the introduction of the Hib booster campaign in 2003.
Of the 36 isolates in the first half of 2022:
- 19 were non-typable
- two were identified as type f
- one was identified as type a
- typing was not carried out for the remaining 14 isolates
Vaccine uptake statistics
Vaccine uptake statistics are published in our childhood immunisation statistics quarterly report.
Measles
Background Information
Before vaccination, measles was a very common childhood disease in Scotland and deaths attributable to measles were substantial.
Following the introduction of measles vaccine in 1968 and the subsequent introduction of the MMR vaccine in 1988, the incidence of the disease has decreased dramatically.
However, as Figure 4 shows, outbreaks have occurred in recent years. These outbreaks have largely occurred in under-immunised populations.
Surveillance update for April to June 2022
One case of laboratory-confirmed measles has been reported in 2022, between January and March. This is the first case reported in Scotland since 2019.
As shown in Figure 5, the number of cases each year has been variable, in:
- 2020 and 2021 there were no cases
- 2019 there were 18 cases
- 2018 there were 2 cases
- 2017 there were 5 cases
- 2016 there were 26 cases
Most cases in Scotland over this time were imported or linked to imported cases within or outwith the UK.
In 2019, there were 18 laboratory-confirmed cases of measles reported.
Of these:
- one case was acquired elsewhere within the UK, outwith Scotland – from which two further
- seven cases were imported from outwith the UK, and resulted in two import-related cases in Scotland
- the remaining six laboratory-confirmed cases of measles in Scotland were of unknown origin – typing indicated that the majority of these cases were strains identified elsewhere in the UK and Europe, which demonstrates transmission within the UK and across the continent prior to the onset of the COVID-19 pandemic
In highly vaccinated populations such as Scotland, it is rare but possible for individuals who have received two doses of MMR vaccine to develop symptoms following exposure to a measles case. However, symptoms are usually attenuated, and individuals are unlikely to be as infectious.
No sustained further transmission occurred in Scotland, highlighting the success of the MMR vaccination programme and the importance of maintaining high vaccine uptake in Scotland.
Of cases reported in 2019, the median age of the 18 laboratory-confirmed cases measles cases was 24 years.
This is similar to the median age of cases in 2016 – which was 22 years – and 2017 – which was 27 years.
Of the two laboratory-confirmed cases reported in 2018 one case was in the under one year age group and the other case reported was in the 30 to 39 year age group. The age distribution of measles cases has varied over recent years, but the majority of cases are observed in children and young adults.
Measles outbreaks occurred across Europe throughout 2018 and continued into 2019.
There has been a notable reduction in cases from March 2020 with this trend likely to be a result of social distancing measures and travel restrictions implemented to limit the spread of COVID-19.
The EU/EEA countries with highest reported rate of cases between this time period were Belgium and Cyprus with a rate of 1.82 and 1.13 cases per million of population, respectively.
Ongoing measles activity in Europe and globally poses a threat to international travellers and Scotland will continue to face an elevated risk of imported cases from other counties and other regions of the UK.
Vaccine uptake statistics
Vaccine uptake statistics are published in our childhood immunisation statistics quarterly report.
Meningococcal Disease
Background information
In 1999, the Meningococcal Invasive Disease Augmented Surveillance (MIDAS) system was introduced.
The surveillance scheme is managed jointly by Public Health Scotland and the Scottish Haemophilus Legionella Meningococcus and Pneumococcus Reference Laboratory (SHLMPRL).
Surveillance data from MIDAS informs the epidemiology of meningococcal disease in Scotland, as analyses can be conducted according to:
- age
- serogroup
- molecular typing
- clinical presentation
- outcome
Surveillance update for April to June 2022
There were six cases of meningococcal disease reported in the second quarter of 2022, bringing the total for the year so far to 15.
This is higher than the number of cases reported for the same period in 2021 (n=5), but lower than the number of cases reported in the second quarter for the previous three years (range 24 to 50) as shown in Figure 6.
Figure 7 shows the number of meningococcal disease cases, according to age group and by quarter from 2001 to the end of the second quarter of 2022.
In the first half of 2022 there were:
- three cases aged under one year
- seven cases in the five to 24 years age group
- five cases in those aged 25 years or over
Of the 15 cases of meningococcal disease reported in the first half of 2022:
- twelve were serogroup B
- three cases were based on clinical diagnosis, as shown in Figure 8
Serogroup W cases continue to be reported separately following introduction of the MenACWY immunisation programme in summer 2015. Figure 9 demonstrates a positive impact of the MenACWY vaccine for the eligible population.
There were no serogroup W cases reported in the first half of 2022.
The number of deaths between 2002 and the end of the second quarter of 2022, reported by serogroup is shown in Figure 10, and case fatality ratio is shown in Figure 11.
There were two deaths from meningococcal disease reported in the first half of 2022.
Vaccination information
MenB vaccine
The MenB vaccine was introduced into the routine childhood vaccination programme on 1 September 2015.
All children born from 1 July 2015 were offered the Men B vaccine at eight weeks, 16 weeks and 12 months of age, alongside other routine childhood vaccinations.
A catch-up programme was rolled out for children born after 1 May 2015.
Children born before 1 May 2015 are not eligible to receive the MenB vaccine.
MenC vaccine
The combined Hib and MenC vaccine given in the UK is called Menitorix® and it is included in the UK childhood immunisation schedule, with routine vaccination recommended between 12 and 13 months of age.
Visit NHS Inform for further information about MenC vaccination.
MenACWY vaccine
MenACWY vaccine was recommended by the Joint Committee on Vaccination and Immunisation (JCVI) and offered to 14 to 18-year-olds as a measure to address an increasing number of meningococcal serogroup W cases in this age group.
A phased catch-up programme also ran in Scotland between August 2015 and March 2016.
The vaccine was also offered to students under the age of 25 attending university for the first time from Autumn 2015. MenACWY vaccine continues to be offered routinely to those in secondary school year 3 (S3).
Visit NHS Inform for further information about MenACWY vaccine.
Further information
Find out more on the addition of MenB to routine vaccinations and their impact (external website).
Read a letter from the Chief Medical Officer letter on the changes to the MenC vaccine.
Vaccine uptake statistics
Vaccine uptake statistics are published in our childhood immunisation statistics quarterly report.
Vaccine uptake statistics for the teenage MenACWY vaccine can be found in our teenage booster immunisation statistics Scotland report.
Mumps
Background information
Mumps is a disease resulting from infection by the mumps virus.
It was a common childhood disease prior to the introduction of the MMR vaccine in 1988, with more than 85% of adults having evidence of previous infection.
Following the introduction of vaccination in 1988, the incidence of mumps has substantially decreased. However, from 2004 to 2020, there was a widespread increased incidence of mumps throughout the UK, with the number of laboratory-confirmed cases peaking in 2005.
Cases of mumps are commonly identified by laboratory testing based on positive PCR or IgM serology and reported to PHS. However, many cases of mumps may be diagnosed clinically or go undiagnosed and the reliance on laboratory reports may represent a considerable underestimate of the true rate of disease in the community.
Surveillance update for April to June 2022
There has been one case of mumps reported in 2022 between April and June.
Since April 2020, there has been a substantial reduction in number of cases of mumps reported with only one laboratory-confirmed case of mumps reported in 2021.
As shown in Figure 12, small outbreaks occurred in:
- 2009
- 2012
- 2014 to 2015
At these points in time, most of the individuals affected were adolescents and young adults and had not received two doses of MMR.
As shown in figure 13, there were:
- 385 cases reported in 2017
- 281 cases reported in 2018
- 784 cases reported in 2019
- 864 cases reported in 2020
- One reported case in 2021
- One case reported in 2022 to end of June
In 2020, the majority of reported cases occurred in the first three months, as shown in figure 13. The decrease in reported cases since April 2020 is likely to be a result of social distancing measures and restrictions implemented in response to the COVID-19 pandemic, which will also have interrupted the transmission of mumps. These measures have also reduced attendance to the primary care setting, resulting in reduced opportunity to diagnose cases.
NHS boards experienced clusters of mumps into the first quarter of 2020, occurring mainly in adolescents and young adults. The observed increase in cases in 2019 and early 2020, prior to the implementation of COVID-19 social restriction measures, may have represented poorer initial immune response to the mumps component of the MMR vaccine, waning immunity, or a combination of both within fully and partially vaccinated individuals.
Age distribution of cases
Figure 14 shows that the majority of mumps cases in recent years have been in those aged 17 to 34 years.
Although the vaccination status of cases is not routinely collected, this is consistent with the age groups that are likely to be under-immunised with a mumps-containing vaccine, or for whom there is waning immunity.
The incidence of mumps by age group in 2020, shown in Figure 15, reflects a higher incidence among individuals aged 17 to 20 years compared to other ages (137 cases per 100,000 population).
This was followed by those aged 21 to 24 years (88 cases per 100,000 population).
*Only one laboratory-confirmed case of mumps was reported in 2021 and one case thus far in 2022, therefore only 2020 data has been presented.
Figure 16 shows the variability in the incidence of mumps between NHS Boards in 2020.
The highest incidence in 2020 was observed in:
- NHS Forth Valley – 48 cases per 100,000 population
- NHS Borders – 34 cases per 100,000 population
- NHS Lothian – 25 cases per 100,000 population
*Only one laboratory-confirmed case of mumps was reported in 2021 and only one case thus far in 2022, therefore only 2020 data has been presented.
Limitations
Relying on laboratory reports will represent an underestimate of mumps since there will be cases that are clinically and not laboratory-confirmation and other cases that may not attend healthcare settings for diagnosis.
It should also be noted that differences in sampling and testing practices may account for variation across the NHS boards.
Vaccine uptake statistics
Vaccine uptake statistics are published in our childhood immunisation statistics quarterly report.
Pertussis
Background information
Pertussis (or whooping cough) is an acute bacterial disease of the respiratory tract, resulting from infection with Bordetella pertussis.
It can affect people of all ages. Adolescents and adults tend to suffer with a prolonged cough. Unimmunised infants are more likely to develop complications from pertussis infection which can require hospital treatment and, in severe cases, can be fatal.
As pertussis continues to circulate in Scotland, immunisation of pregnant women is vital. The immunity that young infants will receive from their mother, although very important in the first few weeks of life, offers only short-term protection. Therefore, it is important that infants are vaccinated as part of the routine childhood schedule on time in order to provide longer-term protection.
Surveillance update April to June 2022
There were two cases of pertussis reported from April to June 2022.
Four cases of laboratory-confirmed pertussis disease were reported in 2021.
In 2020, there were 198 laboratory reports of B. pertussis, the majority of which occurred in the first quarter of the year. This reduction is likely to be attributable to social distancing measures implemented to mitigate the transmission of COVID-19.
Figure 17 shows the number of positive laboratory reports of B. pertussis in Scotland from 2012 to the end of June 2022.
In 2012 and 2013, an outbreak occurred in Scotland, with 1,896 and 1,188 laboratory reports of pertussis, respectively.
Since then, the number of reports annually has been lower than those years:
- 533 in 2017
- 443 in 2018
- 746 in 2019
Age breakdown of cases
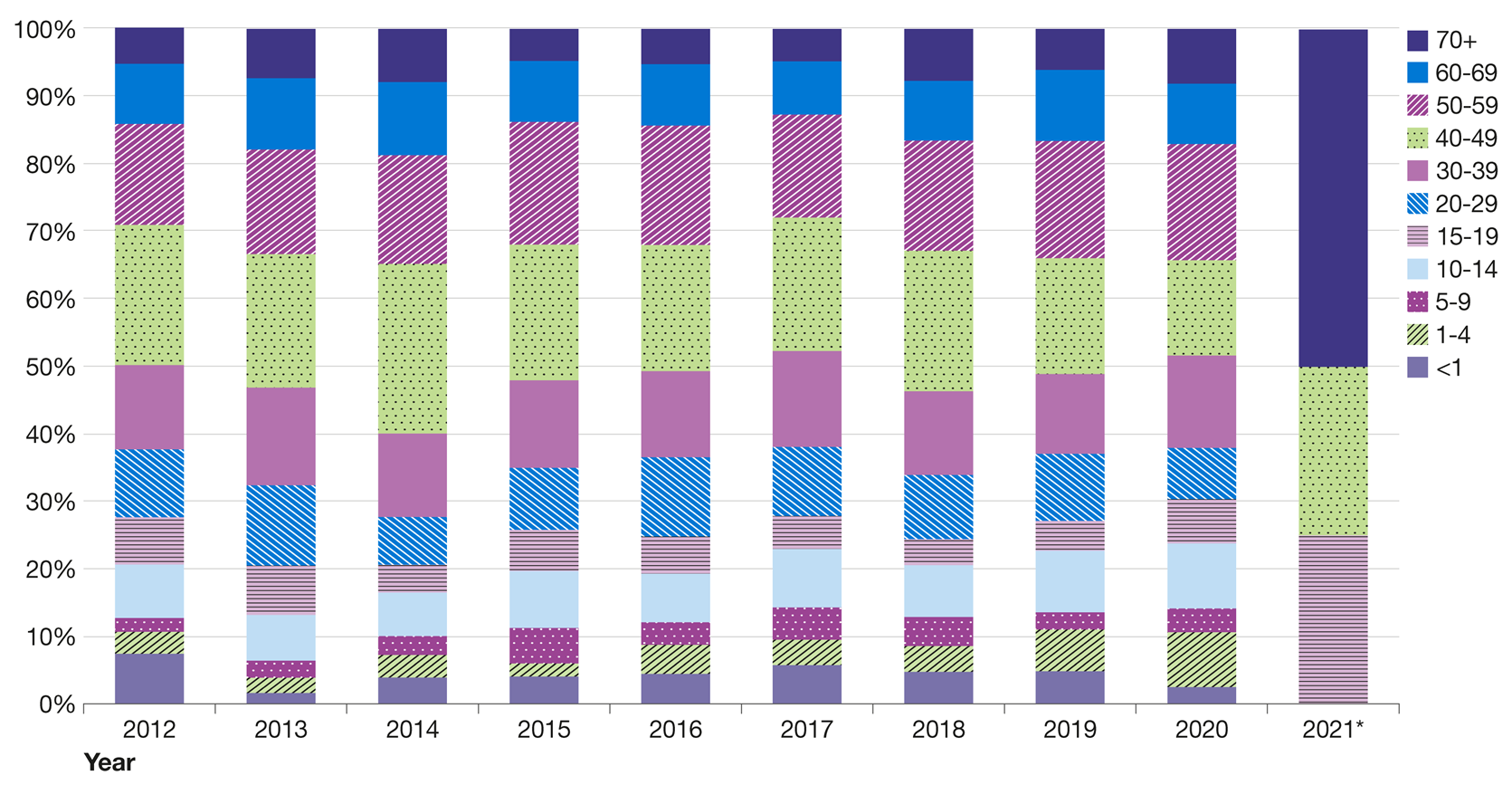
Figure 18 presents the percentage of laboratory reports for pertussis by age group and year from 2012 to the end of December 2021.
The data for 2020 indicate that adults aged 50 to 59 years accounted for a comparatively higher percentage of laboratory reports than individuals in other age groups.
Of the four reported cases in 2021:
- one case was aged between 15 and 19 years
- one case was between 50 to 59 years
- two cases were older than 70 years
Figure 19 presents the number of laboratory reports for pertussis by age group from January to December of 2020.
The graph shows that the 50 to 59 age group accounted for the highest number of cases, however incidence was highest among children under one year of age at 9.8 per 100,000 population as shown in Figure 20.
This was followed by children aged 1 to 4 years, with an incidence of 7.2 per 100,000 population.
*The low number or absence of cases in 2021 and 2022 means that only data until 2020 is presented.
*The low number or absence of cases in 2021 and 2022 means that only data until 2020 is presented.
Laboratory reports by NHS board
NHS Tayside and NHS Greater Glasgow and Clyde each reported one laboratory-confirmed case of pertussis to the end of 2021, with NHS Lothian reporting two cases.
Figure 21 demonstrates the number of pertussis laboratory reports by NHS board from January to December 2020, while Figure 22 presents incidence per 100,000 population.
There is variation between NHS boards, with the highest incidence in 2020 in:
- NHS Highland – 8.1 reports per 100,000 population
- NHS Dumfries – 6.7 reports per 100,000
- NHS Galloway – 6.7 reports per 100,000
No positive laboratory reports were received in 2020 from NHS Orkney, NHS Shetland, and NHS Western Isles.
*The low number/absence of cases in 2021 and 2022 means that only data until 2020 is presented.
*The low number or absence of cases in 2021 and 2022 means that only data until 2020 is presented.
Vaccination information
In response to the increase in cases and to protect young infants in the first few weeks of life until starting the routine childhood immunisation programme at eight weeks, a programme was introduced in October 2012 to offer pertussis vaccination to all pregnant women.
The vaccine is offered to pregnant women between gestational weeks 16 and 32 to maximise protection of the baby from birth.
Women may still be immunised after week 32 of pregnancy but this may not offer as high a level of passive immunological protection to the baby.
Vaccination late in pregnancy may, however, directly protect the mother against disease and thereby reduce the risk of exposure to her infant.
Vaccine uptake statistics
Vaccine uptake statistics are published in our our childhood immunisation statistics quarterly report.
Pneumococcal disease
Background information
Invasive Pneumococcal Disease (IPD) surveillance is based on local and reference laboratory reports confirming isolation of Streptococcus pneumoniae from sterile body sites, mainly blood and cerebrospinal fluid (CSF).
In 1999, the Scottish Pneumococcal Invasive Disease Enhanced Reporting (SPIDER) scheme was introduced. The enhanced surveillance scheme is jointly managed by Public Health Scotland and the Scottish Haemophilus, Legionella, Meningococcus and Pneumococcus Reference Laboratory (SHLMPRL).
Data from SPIDER informs understanding of the epidemiology of IPD in Scotland.
Surveillance update April to June 2022
There were 95 cases of IPD reported in the second quarter of 2022, bringing the total for the first half of the year to 178.
This is higher than the number of cases reported for the same period in 2021 (n=113), but lower than for the first half of the previous three years (range 188 to 340) as shown in Figure 23.
The lower number of cases of IPD observed since early 2020 is likely due to the impact of social distancing measures and other restrictions implemented in response to the COVID-19 pandemic over that period.
Figure 24 presents data on cases by age group, and indicates that the burden of IPD is in adults over 35 years:
- 82 were aged 65 years or older (46.1%)
- 62 were aged 35 to 64 years (34.8%)
- five were aged 15 to 34 years (2.8%)
- three were aged 5 to 14 years (1.7%)
- 26 were aged under five years (14.6%), of whom five were infants aged under one year
IPD in children under five years old
Of the 178 IPD cases reported in the first quarter of 2022, 26 were children under five years of age, 25 of whom were old enough to have been eligible for at least a first dose of PCV13 vaccination.
This is higher than the number of cases reported in children under five for the same period in each of the previous four years, which ranged from 12 to 21.
Serotypes detected in children aged under five years in the first half of 2022 are shown in Table 1.
| serotype | <=2 mths | 3-11 | 1 yr | 2 yrs | 3 yrs | 4 yrs | Total < 5 years |
|---|---|---|---|---|---|---|---|
| 3 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| 7C | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| 10A | 0 | 0 | 1 | 1 | 0 | 0 | 2 |
| 15B | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| 15C | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| 19A | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| 19F | 1 | 0 | 0 | 1 | 0 | 0 | 2 |
| 22F | 0 | 2 | 1 | 0 | 0 | 0 | 3 |
| 23B | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| 33F | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| 38 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| N/A | 0 | 2 | 6 | 1 | 1 | 1 | 11 |
| Total | 1 | 4 | 13 | 4 | 1 | 3 | 26 |
Septicaemia and pneumonia were the most common clinical presentations in children aged under five years old.
Five of the 26 children aged under five years who had IPD in the first half of 2022 were known to have an underlying condition.
Circulating serotypes of Streptococcus pneumoniae
All IPD isolates and specimens should be sent to the reference laboratory for further typing and antimicrobial sensitivities.
Typing results were available for 132 of the 178 cases reported in the first half of 2022. This accounts for 74.2% of the cases reported.
The three most common serotypes reported were:
- Serotype 8 (22 cases)
- Serotype 3 (18 cases)
- Serotype 9N (12 cases)
A total of 33 cases, or 18.5%, were caused by serotypes covered by the PCV13 vaccine.
For the most recent information on antimicrobial resistance in Streptococcus pneumoniae, see Scottish One Health Antimicrobial Use and Antimicrobial Resistance in 2020.
Vaccination information
Two pneumococcal vaccines are available that provide protection against pneumococcal disease.
Pneumococcal polysaccharide vaccine (PPV23)
The pneumococcal polysaccharide vaccine, PPV23, is recommended for many of the same people who receive an annual flu vaccination. Unlike the flu vaccine, which is given every year, the pneumococcal vaccine is usually only given once.
In 2003, the pneumococcal vaccination for all people aged 65 years and over was introduced, in addition to those aged under 65, with certain underlying conditions. This vaccine offers protection against 23 serotypes of IPD.
Pneumococcal conjugate vaccine (PCV7)
In September 2006, the pneumococcal conjugate vaccine PCV7 was introduced into the routine childhood immunisation schedule.
Pneumococcal conjugate vaccine (PCV13)
In spring 2010, PCV7 was replaced with PCV13 to provide broader protection against more serotypes of S. pneumoniae.
PCV13 was initially offered as three doses:
- primary doses at eight weeks
- primary dose at 16 weeks
- booster at 12 to 13 months
From April 2020, the PCV13 schedule changed to:
- primary dose at 12 weeks
- booster dose at between 12 and 13 months.
PCV13 offers protection against the following 13 serotypes of S. pneumoniae:
- 1
- 3
- 4
- 5
- 14
- 6A
- 6B
- 7F
- 9V
- 18C
- 19A
- 19F
- 23F
To coincide with the introduction of PCV7, enhanced surveillance was established for children under five years of age.
A very limited number of high-risk older children and adults are also indicated for PCV13 – see Pneumococcal: the green book, chapter 25 and CMO(2020)02: changes to the childhood pneumococcal conjugate vaccine (PCV13) schedule from 6 April 2020.
Vaccine uptake statistics
Vaccine uptake statistics for children are published in our childhood immunisation statistics quarterly report.
Vaccine uptake statistics for adults are published by our Clinical and Protecting Health Directorate.
Poliomyelitis
Background information
Poliomyelitis (polio) is an acute viral illness caused by one of the three serotypes of poliovirus.
Most infections cause no symptoms, but in a small number of people can result in a potentially life-threatening infection that can cause temporary or permanent paralysis.
People may become infected with the polio virus through contact with infected faecal matter or respiratory secretions.
Immunisation against polio is offered to babies and children as part of the routine childhood immunisation schedule.
They receive:
- three doses of the 6-in-1 vaccine at 8, 12 and 16 weeks of age
- a 4-in-1 pre-school booster at 3 years 4 months old
- a 3-in-1 teenage booster at around 14 years of age (S3)
Guidance on polio immunisation is available in chapter 26 of the UKHSA’s green book.
Surveillance
Following the introduction of the vaccine in the 1950s, the number of cases fell rapidly.
- The last UK case of poliomyelitis caused by wild polio virus was in 1984.
- The last imported case of polio in the UK was 1993.
Poliovirus is targeted by the World Health Organization (WHO) for eradication and, due to the efforts of countries worldwide, polio is now eliminated from four of the six WHO regions.
Pakistan and Afghanistan are considered the countries with the highest risk.
However, polio outbreaks do occur in other countries when the disease is spread amongst people who may not be fully vaccinated.
Visit fitfortravel for more information on those who may be at risk of exposure through travel.
Vaccine uptake statistics
Vaccine uptake statistics for children are published in our childhood immunisation statistics quarterly report.
Rotavirus
Background information
Rotavirus is highly infectious and a leading cause of gastroenteritis in children worldwide. In Scotland, most children will have had at least one rotavirus infection by five years old.
Prior to the implementation of the rotavirus vaccination programme, rotavirus reports peaked between February and April. This caused considerable additional pressure on the NHS, particularly in primary care and paediatric healthcare facilities.
In July 2013, Rotarix®, a live attenuated vaccine was introduced into the routine infant vaccination schedule in the UK, with doses given at 8 and 12 weeks.
Surveillance update April to June 2022
Figure 25 shows the number of rotavirus laboratory reports in Scotland from 2011 to the end of June 2022.
Following the introduction of the immunisation programme, there has been a marked reduction in the number of laboratory reports, which clearly demonstrates the impact of the vaccine.
A reduction of laboratory-confirmed rotavirus samples has also been seen in unvaccinated children suggestive of indirect population protection due to the vaccine.
Hospitalisations
Scottish Morbidity Record 01 (SMR01) is a national dataset held by Public Health Scotland that provides data on admissions to General Acute Inpatient and Day Case Units.
Surveillance of hospital admissions attributable to rotavirus is undertaken using defined International Classification of Disease 10 (ICD-10) codes for rotavirus and viral enteritis (possible rotavirus).
Figure 26 shows the number of admissions for rotavirus for children aged less than five years by month in the three-year period 2014 to 2017 compared to an average of the years 2010 to 2013.
This figure shows the impact of the vaccine on rotavirus related hospital admissions of young children.
The increase in hospital admissions observed in November and December of 2016, was prior to the rotavirus season and therefore most likely associated with increased circulation of norovirus at that time.
GP consultations
Infections resulting in hospitalisation represent a fraction of cases that occur in the community which, while less severe, cause substantial illness.
Methods to monitor the impact of the vaccine programme in community settings have also been established to obtain a more complete representation of the impact of the vaccine on the burden of disease.
Figure 27 presents the consultation rate per 100,000 of the population for infants less than one year of age for gastrointestinal illness and compares 2014 to 2018 to an average of 2010 to 2013 before the vaccine was introduced.
The impact of the vaccine is clear with an absence of a seasonal peak of consultations corresponding to the rotavirus season in the years 2014 to 2018.
Vaccination information
A national rotavirus vaccination programme using the vaccine Rotarix® was introduced into the routine infant vaccination schedule in Scotland in July 2013, with approximately 93% uptake rate.
The vaccine provides protection against the most common strains of rotavirus, but not other enteric viruses such as norovirus.
Infants are offered two doses of the oral vaccine Rotarix®, which is a weakened form of virus which can't cause disease but that protects against rotavirus at an interval of at least four weeks between doses - at eight weeks and again at 12 weeks.
Vaccine uptake statistics
Vaccine uptake statistics are published in our childhood immunisation statistics quarterly report.
Rubella
Background information
Before the introduction of rubella vaccination, more than 80% of adults had evidence of previous exposure to rubella.
A vaccination programme targeting girls and non-immune women of childbearing age was introduced in the UK in 1970 and reduced the number of congenital rubella syndrome (CRS)-related births and terminations.
In 1988, the Measles, Mumps and Rubella (MMR) vaccine was introduced for both boys and girls and further decreased cases of rubella to near elimination levels (Figure 28).
Surveillance update April to May 2022
No laboratory-confirmed cases of rubella were reported between January and June 2022.
The last reported case of laboratory-confirmed rubella in Scotland was in 2017.
Congenital rubella surveillance
Congenital rubella surveillance can be viewed on the Royal College of Paediatrics and Child Health (RCPCH) website.
Vaccine uptake statistics
Vaccine uptake statistics are published in the childhood immunisation statistics quarterly report.
Shingles
Background information
Shingles, also known as herpes zoster, is caused by reactivation of latent varicella zoster virus. Varicella zoster is the same virus that causes chickenpox.
Shingles is characterised by a painful skin rash. The main complication from shingles is post-herpetic neuralgia (PHN), a long-lasting neuropathic pain after the rash has disappeared.
PHN can persist for months or years and the risk and severity increases with age. Its effect can be very debilitating.
The Scottish Morbidity Record 01 (SMR01) is a national dataset held by the NHS Information Services Division and provides data on inpatient and day case admissions. It is used to investigate the burden of disease on hospital inpatient and day-case discharges from acute specialties from hospitals in Scotland.
Shingles surveillance data
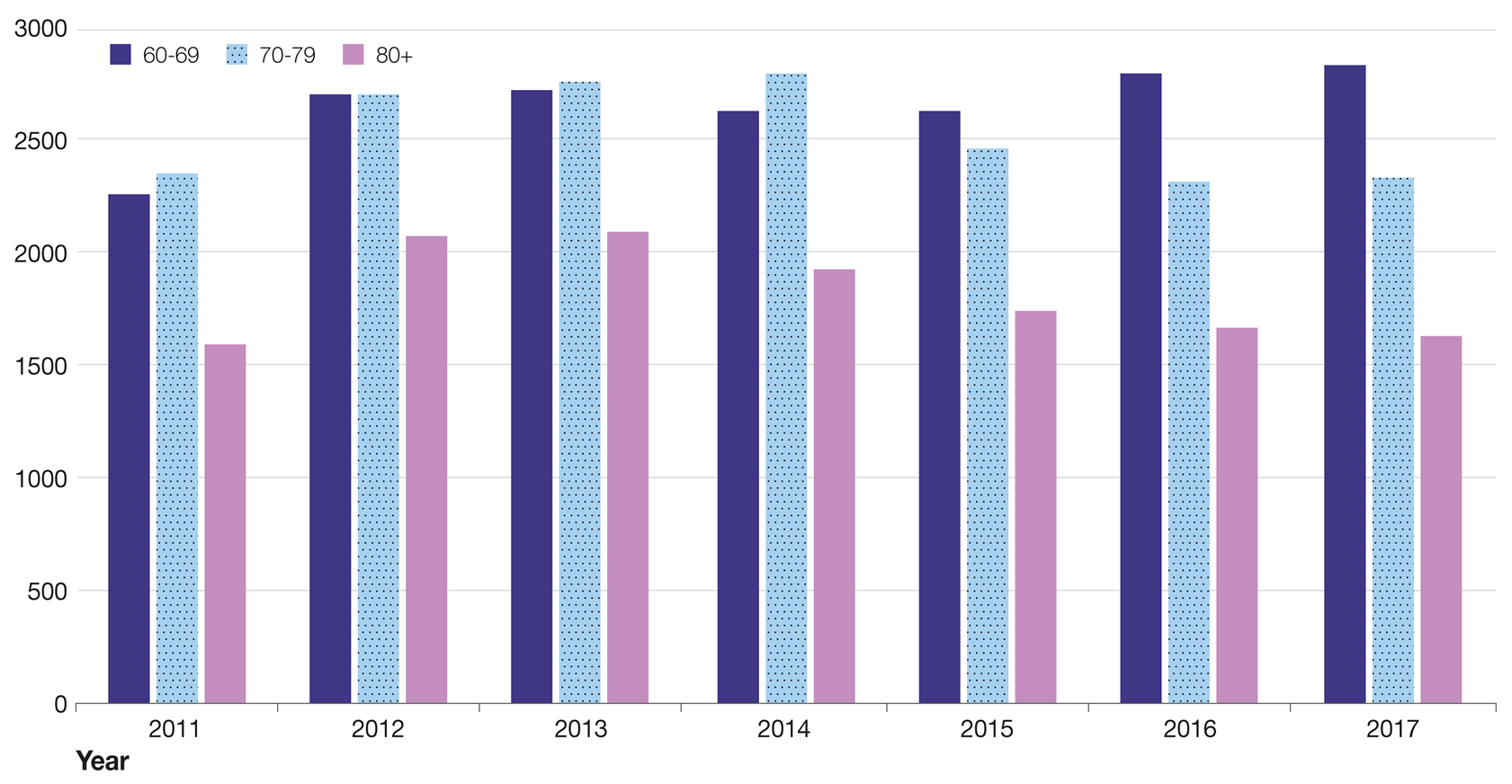
Figure 29 shows the rate of admissions per 100,000 population for shingles and related complications by age group between 2010 and 2021.
This graph shows that the rate of admissions is higher among the older age groups.
*Rates are calculated using an updated methodology, which differs from that previously used to estimate burden of disease. This is therefore not comparable to graphs in previous reports.
GP consultations
An aggregated dataset is received quarterly from approximately 50% of GP practices in Scotland on consultations for shingles and related complications.
Figure 30 presents the number of consultations for shingles by age group between 2011 and 2017.
Figure 31 shows the rate of GP consultations for shingles per 1,000 population by age group between 2011 and 2017.
As with the data in Figure 29, this chart suggests that the burden of shingles is higher among the older age groups with this data demonstrating a decline in the number of consultations after 2014 observed in those over 70 years.
This is consistent with the cohort who were eligible to receive shingles vaccination from September 2013.
Vaccination information
The vaccine is offered routinely to those aged 70 years.
Opportunistic vaccination is offered to eligible individuals aged 71 to 79 years who have not previously been vaccinated.
Read more in Shingles (herpes zoster): the green book, chapter 28a.
Zostavax®
In September 2013, a national shingles vaccination programme was introduced using Zostavax®.
As Zostavax® is a live attenuated vaccine, it cannot be given to patients who are severely immunosuppressed.
Download the screening tool to identify when the Zostavax® vaccine is contraindicated.
Shingrix®
From September 2021 a non-live vaccine was introduced following recommendation from the JCVI.
This vaccine, Shingrix®, should be offered to those people for whom Zostavax® is contraindicated due to severe immunosuppression but who are eligible for vaccination under the current programme. This means that they can gain a similar level of protection to those with no contraindications.
Vaccine uptake statistics
Tables 2 and 3 present shingles vaccine coverage among the eligible routine and catch-up cohort of the Zostavax® vaccine in Scotland for the current programme to the end of May 2022.
- Vaccine coverage among the routine cohort – those aged 70 between 1 September 2021 and 31 August 2022 – was 24.4%.
- Within the catch-up cohort – those aged between 71 and 79 in the same time period – nearly two thirds, or 57.9%, of those eligible have ever received their vaccination against shingles.
The uptake for the 2020 to 2021 season is lower than anticipated.
This trend is likely attributable to the social restriction measures and reduced physical consultation time between patients and clinicians that was implemented to mitigate the spread of COVID-19 infection.
| NHS Board | Number individuals in routine cohort | Number of individuals in routine cohort vaccinated | Shingles vaccination coverage amongst routine cohort (%) |
|---|---|---|---|
| Ayrshire & Arran | 4,522 | 1250 | 27.64 |
| Borders | 1,502 | 118 | 7.86 |
| Dumfries & Galloway | 2,083 | 22 | 1.06 |
| Fife | 4,056 | 1080 | 26.63 |
| Forth Valley | 3,392 | 1193 | 35.17 |
| Grampian | 5,965 | 1054 | 17.67 |
| Greater Glasgow & Clyde | 10,793 | 2,140 | 19.83 |
| Highland | 4,063 | 1245 | 30.64 |
| Lanarkshire | 6,657 | 1,462 | 21.96 |
| Lothian | 8,164 | 1,952 | 23.91 |
| Orkney | 282 | 110 | 39.01 |
| Shetland | 267 | 94 | 35.21 |
| Tayside | 4,735 | 1,582 | 33.41 |
| Western Isles | 349 | 216 | 61.89 |
| Scotland | 56,830 | 13,836 | 24.35 |
*The figures in this column are based on the number of individuals registered at a GP practice and may thus be slightly lower than the overall population in this age group.
| NHS Board | Number of individuals | Number of individuals vaccinated | Shingles vaccination coverage (%) |
|---|---|---|---|
| Ayrshire & Arran | 35,632 | 19538 | 54.83 |
| Borders | 11,962 | 6881 | 57.52 |
| Dumfries & Galloway | 16,533 | 8996 | 54.41 |
| Fife | 32,074 | 19235 | 59.97 |
| Forth Valley | 25,486 | 15708 | 61.63 |
| Grampian | 44,210 | 25123 | 56.83 |
| Greater Glasgow & Clyde | 77,964 | 41,324 | 53.00 |
| Highland | 30,751 | 18986 | 61.74 |
| Lanarkshire | 49,359 | 26,097 | 52.87 |
| Lothian | 61,637 | 37,104 | 60.20 |
| Orkney | 2185 | 1600 | 73.23 |
| Shetland | 2052 | 1246 | 60.72 |
| Tayside | 37,057 | 23,550 | 63.55 |
| Western Isles | 2589 | 1755 | 67.79 |
| Scotland | 429,491 | 248,531 | 57.87 |
*The figures in this column are based on the number of individuals registered at a GP practice and may thus be slightly lower than the overall population in this age group.
Tetanus
Background information
Tetanus is a disease resulting from infection with the bacteria Clostridium tetani.
These bacteria are common in the environment and are present in soil and the manure of animals.
They may cause infection by producing a neurotoxin when they enter the body through a wound, burn, puncture or scratch.
The most common symptoms of infection are:
- lockjaw
- muscle spasms
- fever
- sweating
- tachycardia (high heart rate)
If not treated, symptoms can get worse over the following hours and days.
Tetanus cannot spread from person to person although people who inject drugs (PWID) are at increased risk of infection, through sharing contaminated objects such as needles. Clusters of infection have been previously reported in PWID.
Immunisation against tetanus is the most effective method of prevention. It has been part of the childhood immunisation schedule since 1961.
The immunisation is offered to babies and children who receive:
- three doses of the 6-in-1 vaccine at 8,12 and 16 weeks of age
- a 4-in-1 pre-school booster at 3 years 4 months old
- a 3-in-1 teenage booster at around 14 years of age (S3)
Guidance on tetanus immunisation is available in chapter 30 of the UKHSA’s green book.
Surveillance
View UKHSA data on the annual reported cases of tetanus in England.
Vaccine uptake statistics
Vaccine uptake statistics for children are published in our childhood immunisation statistics quarterly report.
Contacts
General enquiries
If you have an enquiry relating to this publication, please email phs.immunisation@phs.scot.
Media enquiries
If you have a media enquiry relating to this publication, please contact the Communications and Engagement team.
Requesting other formats and reporting issues
If you require publications or documents in other formats, please email phs.otherformats@phs.scot.
To report any issues with a publication, please email phs.generalpublications@phs.scot.
Further information
Statistical designation
This is Official Statistics publication
It is important that users understand that limitations may apply to the interpretation of this data, further details of which are presented in this report.
All official statistics should comply with the UK Statistics Authority’s Code of Practice which promotes the production and dissemination of official statistics that inform decision making.
Visit the UK Statistics Authority UK website to find out more about the Code of Practice (external website).
Find out more about National Statistics on the UK Statistics Authority website (external website).
Early access
Under terms of the "Pre-Release Access to Official Statistics (Scotland) Order 2008", PHS is obliged to publish information on those receiving Pre-Release Access ("Pre-Release Access" refers to statistics in their final form prior to publication). The standard maximum Pre-Release Access is five working days.
Shown below are details of those receiving standard Pre-Release Access.
Standard Pre-Release Access:
- Scottish Government Health Department
- NHS Board Chief Executives
- NHS Board Communication leads
Metadata
The metadata for this document has been split into sections as there are some differences between the diseases.
- Publication title
-
Immunisation and vaccine-preventable diseases quarterly report.
- Description
-
This release provides information on diptheria infections in Scotland for 2022
- Theme
-
Infections in Scotland
- Topic
-
Diphtheria
- Format
-
HTML
- Data source(s)
-
ECOSS (Electronic Communication of Surveillance in Scotland).
- Date that data are acquired
-
1 August 2022.
Agreed date to allow quality assurance checks.
- Release date
-
6 September 2022
- Frequency
-
Quarterly
- Timeframe of data and timeliness
-
April to June 2022, approximately 2 months in arrears
- Continuity of data
-
Quarterly as at March, June, September and December.
Data from 1988 to June 2022 is presented.
- Revisions statement
-
Data in the most recent quarterly updates supersedes data reported in previous reports.
- Revisions relevant to this publication
-
This publication has no revisions.
- Concepts and definitions
-
Diphtheria is an acute bacterial infection affecting the upper respiratory tract or the skin, caused by the diphtheria toxin produced by toxigenic strains of Corynebacterium.
The most common symptoms of diphtheria affecting the upper respiratory tract are membranous pharyngitis with fever, lymphadenopathy and upper respiratory tract soft tissue swelling ‘bull neck’ potentially leading to life-threatening airway obstruction.
Cutaneous diphtheria may cause pus-filled blisters on legs, hands and feet and ulceration of the skin.
In unvaccinated or partially vaccinated individuals, systemic absorption of the toxin can lead to late complications such as cardiac and neurological conditions and sometimes death.
Immunisation against diphtheria is offered to babies and children as part of the routine childhood immunisation schedule.
- Relevance and key uses of the statistics
-
Data are collected as part of mandatory public health surveillance providing data to monitor the epidemiology of Diphtheria and inform public health planning and response.
Statistics are used by PHS for surveillance purposes and published for transparency.
- Accuracy
-
The data are considered accurate.
Data are validated locally by partnerships.
We carry out further validation checks in consultation with NHS boards, as required.
The Code of Practice for Statistics has been followed to ensure a high standard of data value, trustworthiness and quality.
- Completeness
-
All data returned from ECOSS and the enhanced surveillance database are used for analysis
- Comparability
-
Scottish data is regularly compared to UKHSA diphtheria data and information.
- Accessibility
-
It is the policy of PHS to make its websites and products accessible according to our accessibility statement. Graphs and tables have been assessed against PHS accessibility standards.
Accessibility of the report and findings are of continuous consideration throughout the report development.
- Coherence and clarity
-
The report has been produced using the standard PHS publications template and is available as HTML web pages.
- Value type and unit of measurement
-
Number of laboratory-confirmed toxigenic strains of Corynebacterium.
- Disclosure
-
Our protocol on statistical disclosure is followed.
- Official Statistics designation
-
Official Statistics
- UK Statistics Authority Assessment
-
Not assessed.
- Last published
-
7 June 2022
- Next published
-
6 December 2022
- Date of first publication
-
17 March 2020
- Help email
- Date form completed
-
22 August 2022
- Publication title
-
Immunisation and vaccine-preventable diseases quarterly report.
- Description
-
This release provides information on laboratory-confirmed cases of invasive Haemophilus influenzae infections reported in Scotland for the period April to June 2022
- Theme
-
Infections in Scotland
- Topic
-
Haemophilus influenzae
- Format
-
HTML
- Data source(s)
-
ECOSS (Electronic Communication of Surveillance in Scotland).
Enhanced surveillance database for all paediatric (younger than 5 years of age) of any type, and all invasive type b.
- Date that data are acquired
-
1 August 2022.
Agreed date to allow quality assurance checks.
- Release date
-
6 September 2022
- Frequency
-
Quarterly
- Timeframe of data and timeliness
-
April to June 2022, approximately 2 months in arrears
- Continuity of data
-
Quarterly as at March, June, September and December.
Data from 1988 to June 2022 is presented.
- Revisions statement
-
Data in the most recent quarterly updates supersedes data reported in previous reports.
- Revisions relevant to this publication
-
This publication has no revisions.
- Concepts and definitions
-
Haemophilus influenzae (H. influenzae) are bacteria commonly carried in the respiratory tract which can cause serious invasive disease, especially in young children.
Invasive disease is usually caused by the encapsulated strains, specifically, six caspular serotypes (a to f) of which type b (Hib) was the most common, until the introduction of the vaccine.
The most common presentations of invasive H. influenzae infection are meningitis, sepicaemia and acute respiratory infections.
Vaccination for Hib is part of the routine childhood immunisations schedule.
- Relevance and key uses of the statistics
-
Data are collected as part of mandatory public health surveillance providing data to monitor the epidemiology of invasive Haemophilus influenzae and inform public health planning and response.
Statistics are used by PHS for surveillance purposes and published for transparency.
- Accuracy
-
The data are considered accurate.
Data are validated locally by partnerships.
We carry out further validation checks in consultation with NHS boards, as required.
- Completeness
-
All data returned from ECOSS and the enhanced surveillance database are used for analysis
- Comparability
-
Scottish data is regularly compared to UKHSA Haemophilus influenzae data and information.
- Accessibility
-
It is the policy of PHS to make its websites and products accessible according to our accessibility statement. Graphs and tables have been assessed against PHS accessibility standards.
Accessibility of the report and findings are of continuous consideration throughout the report development.
- Coherence and clarity
-
The report has been produced using the standard PHS publications template and is available as HTML web pages.
- Value type and unit of measurement
-
Number of new H. Influenzae isolates from sterile sites.
- Disclosure
-
Our protocol on statistical disclosure is followed.
- Official Statistics designation
-
Official Statistics
- UK Statistics Authority Assessment
-
Not assessed.
- Last published
-
7 June 2022
- Next published
-
6 December 2022
- Date of first publication
-
17 March 2020
- Help email
- Date form completed
-
22 August 2022
- Publication title
-
Immunisation and vaccine-preventable diseases quarterly report.
- Description
-
This release provides information on laboratory-confirmed and epidemiologically linked cases of measles reported in Scotland from April to June 2022
- Theme
-
Infections in Scotland
- Topic
-
Measles infection
- Format
-
HTML
- Data source(s)
-
ECOSS (Electronic Communication of Surveillance in Scotland), Colindale/PHE, Enhanced surveillance database.
- Date that data are acquired
-
1 August 2022.
Agreed date to allow quality assurance checks.
- Release date
-
6 September 2022
- Frequency
-
Quarterly
- Timeframe of data and timeliness
-
April to June 2022, approximately 2 months in arrears
- Continuity of data
-
Quarterly as at March, June, September and December.
Data from 1988 to June 2022 is presented.
- Revisions statement
-
Data in the most recent quarterly updates supersedes data reported in previous reports.
- Revisions relevant to this publication
-
This publication has no revisions.
- Concepts and definitions
-
Measles is a rash illness resulting from infection with the measles virus.
It can affect people of all ages but infants less than one year of age and those who are immunocompromised are at increased risk of complications and death.
It's one of the most communicable diseases with one case having the potential to infect another 12 to 18 individuals through airborne transmission and respiratory droplets in susceptible populations.
New cases of measles are identified by laboratory testing based on positive PCR or IgM serology.
MMR is the combined vaccine that protects against measles, mumps and rubella and is the most effective strategy for preventing the transmission of measles.
- Relevance and key uses of the statistics
-
Data are collected as part of mandatory public health surveillance providing data to monitor the epidemiology of invasive Haemophilus influenzae and inform public health planning and response.
Statistics are used by PHS for surveillance purposes and published for transparency.
- Accuracy
-
The data are considered accurate.
Data are validated locally by partnerships.
We carry out further validation checks in consultation with NHS boards, as required.
- Completeness
-
All data returned from ECOSS and the enhanced surveillance database are used for analysis
- Comparability
-
Scottish data is regularly compared to UKHSA measles data and information.
- Accessibility
-
It is the policy of PHS to make its websites and products accessible according to our accessibility statement. Graphs and tables have been assessed against PHS accessibility standards.
Accessibility of the report and findings are of continuous consideration throughout the report development.
- Coherence and clarity
-
The report has been produced using the standard PHS publications template and is available as HTML web pages.
- Value type and unit of measurement
-
Number of new measles infections
- Disclosure
-
Our protocol on statistical disclosure is followed.
- Official Statistics designation
-
Official Statistics
- UK Statistics Authority Assessment
-
Not assessed.
- Last published
-
7 June 2022
- Next published
-
6 December 2022
- Date of first publication
-
17 March 2020
- Help email
- Date form completed
-
22 August 2022
- Publication title
-
Immunisation and vaccine-preventable diseases quarterly report.
- Description
-
This release provides information on the clinical and laboratory confirmed cases of meningococcal disease reported in Scotland for the period April to June 2022.
- Theme
-
Infections in Scotland
- Topic
-
Meningococcal Disease
- Format
-
HTML
- Data source(s)
-
ECOSS (Electronic Communication of Surveillance in Scotland).
Meningococcal Invasive Disease Augments Surveillance (MIDAS).
- Date that data are acquired
-
1 August 2022.
Agreed date to allow quality assurance checks.
- Release date
-
6 September 2022
- Frequency
-
Quarterly
- Timeframe of data and timeliness
-
April to June 2022, approximately 2 months in arrears
- Continuity of data
-
Quarterly as at March, June, September and December.
Data from 1999 to June 2022 is presented.
- Revisions statement
-
Data in the most recent quarterly updates supersedes data reported in previous reports.
- Revisions relevant to this publication
-
This publication has no revisions.
- Concepts and definitions
-
Meningococcal disease occurs as a result of invasive bacterial infection with the organism Neisseria meningitidis.
Meningococcal disease cases overwhelmingly show symptoms of meningitis (inflammation of the meninges) or septicaemia (blood poisoning).
Meningococcal disease is a significant cause of morbidity and mortality in children and young adults.
N. meningitidis is classified according to its outer membrane characteristics via a process known as serogrouping. There are a number of different serogroups, the most common of which in the UK is B followed by W. Cases of serogroup Y, Z and C disease have also been also reported. Currently there are vaccines to protect against certain strains within serogrouups A, B, C, W and Y.
- Relevance and key uses of the statistics
-
Data are collected as part of mandatory public health surveillance providing data to monitor the epidemiology of meningococcal disease and inform public health planning and response.
Statistics are used by PHS for surveillance purposes and published for transparency.
- Accuracy
-
The data are considered accurate.
Data are validated locally by partnerships.
We carry out further validation checks in consultation with NHS boards, as required.
The Code of Practice for Statistics has been followed to ensure a high standard of data value, trustworthiness and quality.
- Completeness
-
All data returned from ECOSS are used for analysis.
- Comparability
-
Scottish data is regularly compared to UKHSA meningococcal disease data and information.
- Accessibility
-
It is the policy of PHS to make its websites and products accessible according to our accessibility statement. Graphs and tables have been assessed against PHS accessibility standards.
Accessibility of the report and findings are of continuous consideration throughout the report development.
- Coherence and clarity
-
The report has been produced using the standard PHS publications template and is available as HTML web pages.
- Value type and unit of measurement
-
Number of new menigococcal infections.
- Disclosure
-
Our protocol on statistical disclosure is followed.
- Official Statistics designation
-
Official Statistics
- UK Statistics Authority Assessment
-
Not assessed.
- Last published
-
7 June 2022
- Next published
-
6 December 2022
- Date of first publication
-
17 March 2020
- Help email
- Date form completed
-
22 August 2022
- Publication title
-
Immunisation and vaccine-preventable diseases quarterly report.
- Description
-
This release provides information on laboratory-confirmed cases of mumps reported in Scotland for the period from April to June 2022
- Theme
-
Infections in Scotland
- Topic
-
Mumps infection
- Format
-
HTML
- Data source(s)
-
ECOSS (Electronic Communication of Surveillance in Scotland).
- Date that data are acquired
-
1 August 2022.
Agreed date to allow quality assurance checks.
- Release date
-
6 September 2022
- Frequency
-
Quarterly
- Timeframe of data and timeliness
-
April to June 2022, approximately 2 months in arrears
- Continuity of data
-
Quarterly as at March, June, September and December.
Data from 2000 to June 2022 is presented.
- Revisions statement
-
Data in the most recent quarterly updates supersedes data reported in previous reports.
- Revisions relevant to this publication
-
This publication has no revisions.
- Concepts and definitions
-
Mumps is a disease resulting from infection by the mumps virus.
The disease is characterised by swelling of one or both cheeks or sides of the jaw, also known as parotitis, along with fever, headache and swollen glands although asymptomatic mumps infection is common, particularly in children.
Mumps is rarely fatal.
New cases of mumps included in the report are identified by laboratory testing based on positive PCR or IgM serology.
It is important to note that mumps may be diagnosed clnically and only laboratory-confirmed cases are included in the report.
Therefore the data presented may represent an underestimate of the true community circulation of mumps
- Relevance and key uses of the statistics
-
Data are collected as part of mandatory public health surveillance providing data to monitor the epidemiology of mumps and inform public health planning and response.
Statistics are used by PHS for surveillance purposes and published for transparency.
- Accuracy
-
The data are considered accurate.
Data are validated locally by partnerships.
We carry out further validation checks in consultation with NHS boards, as required.
The Code of Practice for Statistics has been followed to ensure a high standard of data value, trustworthiness and quality.
- Completeness
-
All data returned from ECOSS are used for analysis.
- Comparability
-
Scottish data is regularly compared to UKHSA mumps data and information.
- Accessibility
-
It is the policy of PHS to make its websites and products accessible according to our accessibility statement. Graphs and tables have been assessed against PHS accessibility standards.
Accessibility of the report and findings are of continuous consideration throughout the report development.
- Coherence and clarity
-
The report has been produced using the standard PHS publications template and is available as HTML web pages.
- Value type and unit of measurement
-
Number of new mumps infections.
- Disclosure
-
Our protocol on statistical disclosure is followed.
- Official Statistics designation
-
Official Statistics
- UK Statistics Authority Assessment
-
Not assessed.
- Last published
-
7 June 2022
- Next published
-
6 December 2022
- Date of first publication
-
17 March 2020
- Help email
- Date form completed
-
22 August 2022
- Publication title
-
Immunisation and vaccine-preventable diseases quarterly report.
- Description
-
This report provides epidemiological information on positive laboratory cases of Bordetella pertussis in Scotland for the period April to June 2022.
- Theme
-
Infections in Scotland
- Topic
-
Whooping cough
- Format
-
HTML
- Data source(s)
-
Electronic Communication of Surveillance in Scotland (ECOSS) for laboratory reports.
General practice IT systems for maternal pertussis vaccination data.
National Records of Scotland for mid-year population estimates (used for incidence calculations).
- Date that data are acquired
-
1 August 2022.
Agreed date to allow quality assurance checks.
- Release date
-
6 September 2022
- Frequency
-
Quarterly
- Timeframe of data and timeliness
-
April to June 2022, approximately 2 months in arrears.
- Continuity of data
-
Electronic reporting of Bordetella pertussis lab results began in 2005 and was rolled out incrementally over the following years.
Reporting of maternal pertussis vaccination coverage began in 2012.
- Revisions statement
-
Data in the most recent quarterly updates supersedes data reported in previous reports.
- Revisions relevant to this publication
-
Planned revisions have been made to historical data based on updated laboratory information received through ECOSS.
- Concepts and definitions
-
Whooping cough (or pertussis) is a highly contagious respiratory illness caused by infection with the bacterium Bordetella pertussis.
Pertussis is spread from person to person by coughing and sneezing.
Early symptoms often include a runny nose, fever, and mild cough, which after a few weeks can progress to uncontrolled coughing fits and subsequent vomiting episodes.
Some individuals with pertussis exhibit a characteristic "whoop" sound caused by gasping for breath after coughing fits.
Unimmunised infants are most at risk of severe complications, which include pneumonia, seizures, brain damage, and death.
Vaccination against pertussis is offered to infants at 8, 12, and 16 weeks of age and to children at 3 years and 4 months of age. Vaccination is also offered to all pregnant women between 16 and 32 weeks of gestation.
- Relevance and key uses of the statistics
-
These data are essential for monitoring the epidemiology of pertussis and the uptake of the maternal pertussis vaccine in Scotland in order to inform public health planning and response.
- Accuracy
-
The data are considered accurate.
Data are validated locally by partnerships.
We carry out further validation checks in consultation with NHS boards, as required.
- Completeness
-
Count of pertussis laboratory reports (number).
Incidence of laboratory reports (rate per 100,000 population).
Age breakdown of laboratory reports (percentage). - Comparability
-
Scottish data is regularly compared to UKHSA pertussis data and information.
- Accessibility
-
It is the policy of PHS to make its websites and products accessible according to our accessibility statement. Graphs and tables have been assessed against PHS accessibility standards.
Accessibility of the report and findings are of continuous consideration throughout the report development.
- Coherence and clarity
-
The report has been produced using the standard PHS publications template and is available as HTML web pages.
- Value type and unit of measurement
-
Count of pertussis laboratory reports (number).
Incidence of laboratory reports (rate per 100,000 population).
Age breakdown of laboratory reports (percentage).
- Disclosure
-
Our protocol on statistical disclosure is followed.
- Official Statistics designation
-
Official Statistics
- UK Statistics Authority Assessment
-
Not assessed.
- Last published
-
7 June 2022
- Next published
-
6 December 2022
- Date of first publication
-
17 March 2020
- Help email
- Date form completed
-
22 August 2022
- Publication title
-
Immunisation and vaccine-preventable diseases quarterly report.
- Description
-
This release provides information on poliomyelitis infections in Scotland for 2022
- Theme
-
Infections in Scotland
- Topic
-
Poliomyelitis
- Format
-
HTML
- Data source(s)
-
ECOSS (Electronic Communication of Surveillance in Scotland).
- Date that data are acquired
-
1 August 2022.
Agreed date to allow quality assurance checks.
- Release date
-
6 September 2022
- Frequency
-
Quarterly
- Timeframe of data and timeliness
-
April to June 2022, approximately 2 months in arrears
- Continuity of data
-
Quarterly as at March, June, September and December.
Data from 1988 to June 2022 is presented.
- Revisions statement
-
Data in the most recent quarterly updates supersedes data reported in previous reports.
- Revisions relevant to this publication
-
This publication has no revisions.
- Concepts and definitions
-
Poliomyelitis (polio) is an acute viral illness caused by one of the three serotypes of poliovirus.
Most infections cause no symptoms, but in a small number of people can result in a potentially life-threatening infection that can cause temporary or permanent paralysis.
People may become infected with the polio virus through contact with infected faecal matter or respiratory secretions.
Immunisation against polio is offered to babies and children as part of the routine childhood immunisation schedule.
- Relevance and key uses of the statistics
-
Data are collected as part of mandatory public health surveillance providing data to monitor the epidemiology of poliomyelitis and inform public health planning and response.
Statistics are used by PHS for surveillance purposes and published for transparency.
- Accuracy
-
The data are considered accurate.
Data are validated locally by partnerships.
We carry out further validation checks in consultation with NHS boards, as required.
The Code of Practice for Statistics has been followed to ensure a high standard of data value, trustworthiness and quality.
- Completeness
-
All data returned from ECOSS are used for analysis.
- Comparability
-
Scottish data is regularly compared to UKHSA poliomyelitis data and information.
- Accessibility
-
It is the policy of PHS to make its websites and products accessible according to our accessibility statement. Graphs and tables have been assessed against PHS accessibility standards.
Accessibility of the report and findings are of continuous consideration throughout the report development.
- Coherence and clarity
-
The report has been produced using the standard PHS publications template and is available as HTML web pages.
- Value type and unit of measurement
-
Number of laboratory-confirmed poliovirus infections.
- Disclosure
-
Our protocol on statistical disclosure is followed.
- Official Statistics designation
-
Official Statistics
- UK Statistics Authority Assessment
-
Not assessed.
- Last published
-
7 June 2022
- Next published
-
6 December 2022
- Date of first publication
-
17 March 2020
- Help email
- Date form completed
-
22 August 2022
- Publication title
-
Immunisation and vaccine-preventable diseases quarterly report.
- Description
-
This release provides information on laboratory-confirmed cases of invasive pneumococcal disease reported in Scotland and vaccination uptake for the period April to June 2022
- Theme
-
Infections in Scotland
- Topic
-
Pneumococcal disease
- Format
-
HTML
- Data source(s)
-
ECOSS (Electronic Communication of Surveillance in Scotland), Scottish Pneumococcal invasive disease enhanced reporting (SPIDER) for all paediatric cases (<5 years old)
- Date that data are acquired
-
1 August 2022
Agreed date to allow quality assurance checks.
- Release date
-
6 September 2022
- Frequency
-
Quarterly
- Timeframe of data and timeliness
-
April to June 2022, approximately 2 months in arrears.
- Continuity of data
-
Quarterly as at March, June, September and December.
Data from 1999 to June 2022 is presented.
- Revisions statement
-
Data in the most recent quarterly updates supersedes data reported in previous reports.
- Revisions relevant to this publication
-
This publication has no revisions.
- Concepts and definitions
-
Streptococcus pneumoniae (S. pneumoniae) is the bacterium responsible for causing pneumococcal infection.
Pneumococcal infections are defined as invasive or non-invasive according to which area of the body is affected.
Invasive pneumococcal disease (IPD) is caused by infection of normally sterile sites, for example, blood and cerebrospinal fluid (CSF). IPD is a major cause of morbidity and mortality in the very young, elderly or immunocompromised individuals. Two pneumococcal vaccines are available that help to protect against pneumococcal disease.
New cases of IPD are identified by laboratory reports confirming isolation of S. pneumoniae from sterile body sites.
- Relevance and key uses of the statistics
-
Data are collected as part of mandatory public health surveillance providing data to monitor the epidemiology of meningococcal disease and inform public health planning and response.
Statistics are used by PHS for surveillance purposes and published for transparency.
- Accuracy
-
The data are considered accurate.
Data are validated locally by partnerships.
We carry out further validation checks in consultation with NHS boards, as required.
The Code of Practice for Statistics has been followed to ensure a high standard of data value, trustworthiness and quality.
- Completeness
-
All data returned from ECOSS systems and the enhanced surveillance database are used for analysis.
- Comparability
-
Scottish data is regularly compared to UKHSA pneumococcal data and information.
- Accessibility
-
It is the policy of PHS to make its websites and products accessible according to our accessibility statement. Graphs and tables have been assessed against PHS accessibility standards.
Accessibility of the report and findings are of continuous consideration throughout the report development.
- Coherence and clarity
-
The report has been produced using the standard PHS publications template and is available as HTML web pages.
- Value type and unit of measurement
-
Number of new S. pnuemoniae isolates from sterile sites.
- Disclosure
-
Our protocol on statistical disclosure is followed.
- Official Statistics designation
-
Official Statistics
- UK Statistics Authority Assessment
-
Not assessed.
- Last published
-
7 June 2022
- Next published
-
6 December 2022
- Date of first publication
-
17 March 2020
- Help email
- Date form completed
-
22 August 2022
- Publication title
-
Immunisation and vaccine-preventable diseases quarterly report.
- Description
-
This release provides information on laboratory-confirmed cases of rotavirus to the period June 2022, consultation rate per 100,000 of the population for infants less than one year of age for gastrointestinal illness to the year end 2018, and on hospital admissions attributable to rotavirus to the end of 2017.
- Theme
-
Infections in Scotland
- Topic
-
Rotavirus
- Format
-
HTML
- Data source(s)
-
Electronic Communication of Surveillance in Scotland (ECOSS) for laboratory reports.
Scottish Morbidity Record (SMR01) for hospital admissions for rotavirus and viral enteritis.
General practice IT systems for vaccination data and GP consultations.
National Records of Scotland for mid-year population estimates (used for rate calculations).
- Date that data are acquired
-
1 August 2022.
Agreed date to allow quality assurance checks.
- Release date
-
6 September 2022
- Frequency
-
Quarterly
- Timeframe of data and timeliness
-
April to June 2022, approximately 2 months in arrears.
- Continuity of data
-
Quarterly as at March, June, September and December.
Data from 2011 to March 2022 is presented.
- Revisions statement
-
Data in the most recent quarterly updates supersedes data reported in previous reports.
- Revisions relevant to this publication
-
This publication has no revisions.
- Concepts and definitions
-
Rotavirus infections in children and adults can last approximately three to eight days and symptoms include severe diarrhoea, vomiting, stomach cramps, mild fever.
The combination of symptoms can lead to dehydration, requiring admission to hospital, especially in young infants.
Before the introduction of a national infant rotavirus vaccination programme in 2013, an estimated 55,000 gastroenteritis cases caused by rotavirus occurred in Scotland each year in children less than five years old. Approximately 1,200 of these children were hospitalised.
The vaccine provides protection against the most common strains of rotavirus, but not other enteric viruses such as norovirus.
- Relevance and key uses of the statistics
-
Data are collected as part of mandatory public health surveillance providing data to monitor the epidemiology of rotavirus and inform public health planning and response.
Statistics are used by PHS for surveillance purposes and published for transparency.
- Accuracy
-
The data are considered accurate.
Data are validated locally by partnerships.
We carry out further validation checks in consultation with NHS boards, as required.
The Code of Practice for Statistics has been followed to ensure a high standard of data value, trustworthiness and quality.
- Completeness
-
Hospital admission data is analysed once SMR01 completeness reaches at least 95%.
Information on GP consultations and shingles vaccination coverage is normally based on data received for over 95% of general practices.
- Comparability
-
Scottish data is regularly compared to UKHSA rotavirus data and information.
- Accessibility
-
It is the policy of PHS to make its websites and products accessible according to our accessibility statement. Graphs and tables have been assessed against PHS accessibility standards.
Accessibility of the report and findings are of continuous consideration throughout the report development.
- Coherence and clarity
-
The report has been produced using the standard PHS publications template and is available as HTML web pages.
- Value type and unit of measurement
-
Count of laboratory-confirmed rotavirus (number).
Hospital admissions for rotavirus and viral enteritis in children aged less than 5 years (count).
GP consultations for gastrointestinal illness in children less than one years (rate per 100,000 population).
- Disclosure
-
Our protocol on statistical disclosure is followed.
- Official Statistics designation
-
Official Statistics
- UK Statistics Authority Assessment
-
Not assessed.
- Last published
-
7 June 2022
- Next published
-
6 December 2022
- Date of first publication
-
17 March 2020
- Help email
- Date form completed
-
22 August 2022
- Publication title
-
Immunisation and vaccine-preventable diseases quarterly report.
- Description
-
This release provides information on laboratory-confirmed and epidemiologically cases of rubella reported in Scotland for the period April to June 2022.
- Theme
-
Infections in Scotland
- Topic
-
Rubella infection
- Format
-
HTML
- Data source(s)
-
ECOSS (Electronic Communication of Surveillance in Scotland).
Colindale/PHE enhanced surveillance database.
- Date that data are acquired
-
1 August 2022.
Agreed date to allow quality assurance checks.
- Release date
-
6 September 2022
- Frequency
-
Quarterly
- Timeframe of data and timeliness
-
April to June 2022, approximately 2 months in arrears
- Continuity of data
-
Quarterly as at March, June, September and December.
Data from 1988 to June 2022 is presented.
- Revisions statement
-
Data in the most recent quarterly updates supersedes data reported in previous reports.
- Revisions relevant to this publication
-
This publication has no revisions.
- Concepts and definitions
-
Rubella is a rash illness caused by the rubella virus and is also known as German measles.
It is generally a mild self-limiting illness, but if acquired by women in the first 16 weeks of pregnancy can have devastating effects on the unborn child inlcuding miscarriage or Congenital Rubella Syndrome (CRS).
New cases of rubella are identified by laboratory testing based on positive PCR or IgM serology.
- Relevance and key uses of the statistics
-
Data are collected as part of mandatory public health surveillance providing data to monitor the epidemiology of rubella and inform public health planning and response.
Statistics are used by PHS for surveillance purposes and published for transparency.
- Accuracy
-
The data are considered accurate.
Data are validated locally by partnerships.
We carry out further validation checks in consultation with NHS boards, as required.
The Code of Practice for Statistics has been followed to ensure a high standard of data value, trustworthiness and quality.
- Completeness
-
All data returned from ECOSS and the enhanced surveillance database are used for analysis.
- Comparability
-
Scottish data is regularly compared to UKHSA rubella data and information.
- Accessibility
-
It is the policy of PHS to make its websites and products accessible according to our accessibility statement. Graphs and tables have been assessed against PHS accessibility standards.
Accessibility of the report and findings are of continuous consideration throughout the report development.
- Coherence and clarity
-
The report has been produced using the standard PHS publications template and is available as HTML web pages.
- Value type and unit of measurement
-
Number of new rubella infections.
- Disclosure
-
Our protocol on statistical disclosure is followed.
- Official Statistics designation
-
Official Statistics
- UK Statistics Authority Assessment
-
Not assessed.
- Last published
-
7 June 2022
- Next published
-
6 December 2022
- Date of first publication
-
17 March 2020
- Help email
- Date form completed
-
22 August 2022
- Publication title
-
Immunisation and vaccine-preventable diseases quarterly report.
- Description
-
This report provides information on hospital admissions and GP consultations for shingles and shingles related complications in Scotland which are proxy measures for shingles disease.
Cumulative coverage estimates of shingles vaccination for the current season are also provided.
- Theme
-
Infections in Scotland
- Topic
-
Herpes zoster infection
- Format
-
HTML
- Data source(s)
-
Scottish Morbidity Record (SMR01) for hospital admissions for shingles and related complications.
General practice IT systems for vaccination data and GP consultations.
National Records of Scotland for mid-year population estimates (used for rate calculations).
- Date that data are acquired
-
1 August 2022.
Agreed date to allow quality assurance checks.
- Release date
-
6 September 2022
- Frequency
-
Quarterly
- Timeframe of data and timeliness
-
GP consultation data from 2011 to 2017.
Hospital admission data from 2010 to 2021.
Shingles uptake data covers the period December 2021 to May 2022.
- Continuity of data
-
A national shingles vaccination programme was introduced in Scotland in September 2013.
Data on hospital admissions due to shingles and related complications are provided from 2010 onwards, while data on GP consultations are provided from 2011 onwards.
- Revisions statement
-
Data in the most recent quarterly updates supersedes data reported in previous reports.
- Revisions relevant to this publication
-
This publication has no revisions.
- Concepts and definitions
-
Shingles, also known as herpes zoster, is caused by reactivation of latent varicella zoster virus, which is the same virus that causes chickenpox.
Following initial infection, usually in childhood, the virus can lie inactive in the body’s nervous system.
Reactivation of the virus can take place later in life, when the immune system has been weakened by factors such as age, stress, illness, or immunosuppresant treatments.
Shingles is characterised by a painful skin rash and the primary complication of this illness is post-herpetic neuralgia, a neuropathic pain which can last for months of years after the rash has disappeared.
The shingles vaccine is offered routinely to those aged 70 years and opportunistically to individuals ages 71 to 79 years who have not previously been vaccinated.
- Relevance and key uses of the statistics
-
Data are collected as part of mandatory public health surveillance providing data to monitor the epidemiology of herpes zoster and inform public health planning and response.
Statistics are used by PHS for surveillance purposes and published for transparency.
- Accuracy
-
The data are considered accurate.
Data are validated locally by partnerships.
We carry out further validation checks in consultation with NHS boards, as required.
The Code of Practice for Statistics has been followed to ensure a high standard of data value, trustworthiness and quality.
- Completeness
-
Hospital admission data is analysed once SMR01 completeness reaches at least 95%.
Information on GP consultations and shingles vaccination coverage is normally based on data received for over 95% of general practices.
- Comparability
-
Scottish data is regularly compared to UKHSA shingles data and information.
- Accessibility
-
It is the policy of PHS to make its websites and products accessible according to our accessibility statement. Graphs and tables have been assessed against PHS accessibility standards.
Accessibility of the report and findings are of continuous consideration throughout the report development.
- Coherence and clarity
-
The report has been produced using the standard PHS publications template and is available as HTML web pages.
- Value type and unit of measurement
-
Hospital admissions for shingles and related complications (rate per 100,000 population).
GP consultations for shingles and related complications (rate per 1,000 population).
Count of GP consultations for shingles and related complications (number).
Coverage of shingles vaccination (percentage).
- Disclosure
-
Our protocol on statistical disclosure is followed.
- Official Statistics designation
-
Official Statistics
- UK Statistics Authority Assessment
-
Not assessed.
- Last published
-
7 June 2022
- Next published
-
6 December 2022
- Date of first publication
-
17 March 2020
- Help email
- Date form completed
-
22 August 2022
- Publication title
-
Immunisation and vaccine-preventable diseases quarterly report.
- Description
-
This release provides information on tetanus infections in Scotland for 2022.
- Theme
-
Infections in Scotland
- Topic
-
Tetanus
- Format
-
HTML
- Data source(s)
-
ECOSS (Electronic Communication of Surveillance in Scotland).
- Date that data are acquired
-
1 August 2022.
Agreed date to allow quality assurance checks.
- Release date
-
6 September 2022
- Frequency
-
Quarterly
- Timeframe of data and timeliness
-
April to June 2022, approximately 2 months in arrears
- Continuity of data
-
Quarterly as at March, June, September and December.
Data from 1988 to June 2022 is presented.
- Revisions statement
-
Data in the most recent quarterly updates supersedes data reported in previous reports.
- Revisions relevant to this publication
-
This publication has no revisions.
- Concepts and definitions
-
Tetanus is a disease resulting from infection with the bacteria Clostridium tetani.
These bacteria are common in the environment and are present in soil and the manure of animals.
They may cause infection by producing a neurotoxin when they enter the body through a wound, burn, puncture or scratch.
The most common symptoms of infection are lockjaw, muscle spasms, fever, sweating and tachycardia (high heart rate).
Tetanus cannot spread from person to person although people who inject drugs (PWID) are at increased risk of infection, through sharing contaminated objects such as needles, and clusters of infection have been previously reported in PWID.
Immunisation against tetanus is the most effective method of prevention has been part of the childhood immunisation schedule since 1961.
- Relevance and key uses of the statistics
-
Data are collected as part of mandatory public health surveillance providing data to monitor the epidemiology of poliomyelitis and inform public health planning and response.
Statistics are used by PHS for surveillance purposes and published for transparency.
- Accuracy
-
The data are considered accurate.
Data are validated locally by partnerships.
We carry out further validation checks in consultation with NHS boards, as required.
The Code of Practice for Statistics has been followed to ensure a high standard of data value, trustworthiness and quality.
- Completeness
-
All data returned from ECOSS are used for analysis.
- Comparability
-
Scottish data is regularly compared to UKHSA tetanus data and information.
- Accessibility
-
It is the policy of PHS to make its websites and products accessible according to our accessibility statement. Graphs and tables have been assessed against PHS accessibility standards.
Accessibility of the report and findings are of continuous consideration throughout the report development.
- Coherence and clarity
-
The report has been produced using the standard PHS publications template and is available as HTML web pages.
- Value type and unit of measurement
-
Number of new Clostridium tetani infections.
- Disclosure
-
Our protocol on statistical disclosure is followed.
- Official Statistics designation
-
Official Statistics
- UK Statistics Authority Assessment
-
Not assessed.
- Last published
-
7 June 2022
- Next published
-
6 December 2022
- Date of first publication
-
17 March 2020
- Help email
- Date form completed
-
22 August 2022